Details of the Drug
General Information of Drug (ID: DM26IH8)
| Drug Name |
S-297995
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
NALDEMEDINE; UNII-03KSI6WLXH; 03KSI6WLXH; S-297,995; 916072-89-4; Naldemedine [USAN:INN]; Naldemedine (USAN/INN); S 297995; SCHEMBL9880572; GTPL9150; CHEMBL2105755; AKOS032945757; DB11691; Morphinan-7-carboxamide, 17-(cyclopropylmethyl)-6,7-didehydro-4,5-epoxy-3,6,14- trihydroxy-N-(1-methyl-1-(3-phenyl-1,2,4-oxadiazol-5-yl)ethyl)-,(5alpha)-; J3.573.009E; D10188
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
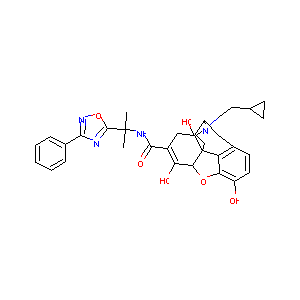 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 570.6 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.2 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 6 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 9 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from S-297995 (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Clinical pipeline report, company report or official report of Shionogi (2011). | ||||
|---|---|---|---|---|---|
| 2 | Product Information. Symproic (naldemedine). Shionogi USA Inc, Florham Park, NJ. | ||||
| 3 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 4 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 5 | Product Information. Balversa (erdafitinib). Janssen Products, LP, Horsham, PA. | ||||
| 6 | Product Information. Turalio (pexidartinib). Daiichi Sankyo, Inc., Parsippany, NJ. | ||||
| 7 | Product Information. Orladeyo (berotralstat). BioCryst Pharmaceuticals Inc, Durham, NC. | ||||
| 8 | Product Information. Tabrecta (capmatinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 9 | Product Information. Reyvow (lasmiditan). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 10 | EMA. European Medicines Agency. European Union "EMA - List of medicines under additional monitoring.". | ||||
| 11 | Product Information. Xeglyze (abametapir topical). Dr. Reddy's Laboratories Inc, Upper Saddle River, NJ. | ||||
| 12 | Product Information. Xenleta (lefamulin). Nabriva Therapeutics US, Inc., King of Prussia, PA. | ||||
| 13 | Product Information. Tavalisse (fostamatinib). Rigel Pharmaceuticals, South San Francisco, CA. | ||||
